Introduction
The production process of Nicotinamide Mononucleotide (NMN) is a complex procedure that involves several key stages, each of which can affect the quality and purity of the final product. As a wholesaler, understanding how NMN is produced can help you ensure that you are sourcing a product that meets your customers’ needs and expectations. In this article, we will break down the steps involved in NMN production, from raw material sourcing to the final product.
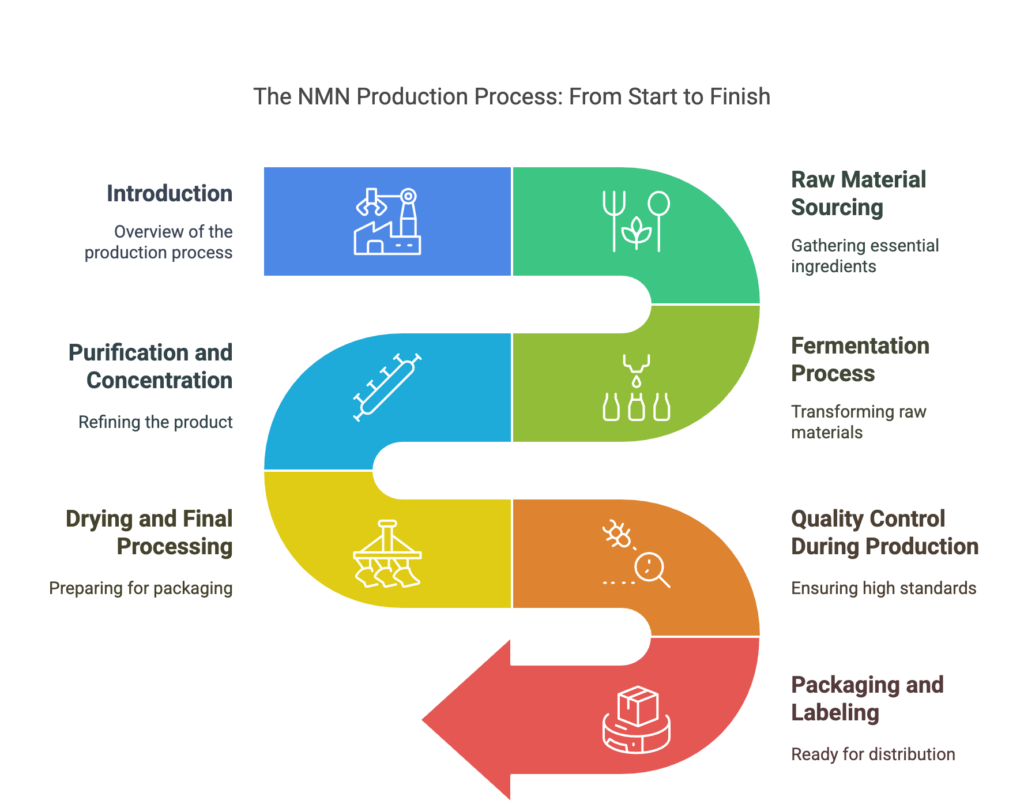
Raw Material Sourcing
The production of NMN begins with the sourcing of high-quality raw materials. NMN is often derived from natural sources, primarily through the process of fermentation. The quality of the raw materials used in production directly influences the final product’s purity and effectiveness.
Fermentation Process
Fermentation is one of the most common methods used to produce NMN. During fermentation, microorganisms such as bacteria or yeast are used to convert simple sugars into NMN. This process is highly controlled to ensure that the final product has a high purity and is free of harmful byproducts.
After fermentation, the NMN is isolated and purified through various techniques, including filtration and crystallization, to remove any contaminants and ensure the product is of the highest quality.
Purification and Concentration
Once NMN is extracted from the fermentation process, it undergoes a series of purification steps to remove any remaining impurities. These may include heavy metals, residual solvents, and microbial contaminants. Purification techniques often include:
- Filtration: To remove particles and impurities.
- Chromatography: A method used to separate compounds and ensure NMN is isolated in its purest form.
Following purification, NMN is concentrated to ensure that the final product has the desired potency. Concentration helps ensure the correct dosage is achieved in the final product, whether in powder, capsule, or liquid form.
Drying and Final Processing
After purification, the concentrated NMN undergoes drying, which turns the liquid or semi-liquid NMN into a powder form or reduces it to a more stable liquid form. This step is crucial because it affects the shelf life and ease of storage of the final product.
Depending on the intended use of the product, NMN may be dried into a powder, encapsulated into soft gels or capsules, or converted into a liquid form. The final processing step ensures the product is ready for packaging and distribution.
Quality Control During Production
Throughout the entire production process, stringent quality control measures must be in place. This includes monitoring the raw material quality, checking purity at each stage of production, and ensuring that the final product meets the required specifications.
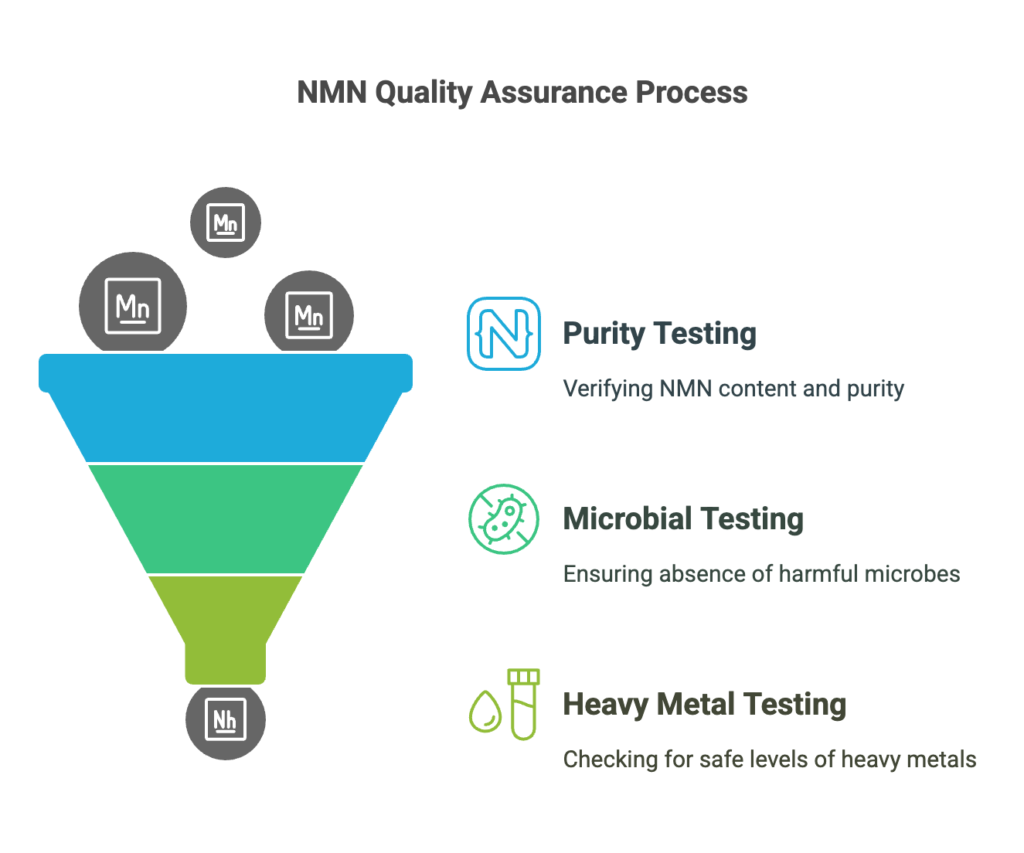
Key quality checks include:
- Purity Testing: Verifying the final product’s NMN content and ensuring it meets the claimed purity (usually 98% or higher).
- Microbial Testing: Ensuring that the product is free from harmful bacteria, fungi, or other contaminants.
- Heavy Metal Testing: Ensuring that the NMN product does not contain harmful levels of heavy metals such as lead, arsenic, or cadmium.
Wholesalers should ensure that suppliers provide third-party testing results for each batch of NMN.
For more on evaluating NMN quality, refer to our article on How to Evaluate NMN Quality: A Wholesaler’s Guide.
Packaging and Labeling
After quality control tests are completed, the final NMN product is packaged and labeled for distribution. Proper packaging ensures the product remains safe and effective during transportation and storage. Labels must comply with industry regulations and include essential information, such as:
- Product Name and Ingredients: Clear identification of the product and its ingredients.
- Batch Number: For traceability and quality assurance.
- Expiration Date: To ensure product freshness and potency.
Wholesalers should ensure that the packaging meets regulatory requirements and the product is correctly labeled.
Conclusion
Understanding the NMN production process is essential for wholesalers who want to source high-quality products. By familiarizing themselves with each stage of production, from raw material sourcing to final packaging, wholesalers can ensure they are sourcing NMN that meets their standards and satisfies their customers’ expectations. Working with suppliers who maintain transparency in their production process and follow rigorous quality control practices is key to building a successful business in the growing NMN market.
To learn more about NMN knowlege, refer to our article on The Ultimate Guide to Nicotinamide Mononucleotide (NMN): A Deep Dive from Quality to Production Process.