
Glutathione Powder Price vs. Quality: How to Make the Right Wholesale Choice
Discover why glutathione powder prices vary and how to evaluate cost vs. quality when sourcing bulk ingredients. Includes quote checklist and supplier comparison tips.

Discover why glutathione powder prices vary and how to evaluate cost vs. quality when sourcing bulk ingredients. Includes quote checklist and supplier comparison tips.

Should you buy in bulk or stay lean? Learn how start-ups and growing brands can align glutathione sourcing strategies to reduce cost and scale effectively.

Learn how to choose between small MOQ and bulk glutathione orders, including pros, cons, hybrid tips, and which strategy aligns with your business growth.

Learn how to verify the authenticity of glutathione powder, avoid oxidized or adulterated batches, and ask your supplier the right questions. Includes COA validation tips.

This article provides a detailed guide on how to interpret a Certificate of Analysis (COA), helping consumers understand key information in the report to ensure the product meets quality and safety standards. The article focuses on how to verify Report Date, Brand Name, Batch Number, and Product Description, as well as how to interpret Safety Test Results for potential harmful substances such as residual solvents, heavy metals, and pathogenic microorganisms. By correctly reading the COA, consumers can confirm the authenticity and safety of the product, making informed purchasing decisions.

This comprehensive guide covers the entire journey of glutathione powder—from raw material sourcing and production to quality control, packaging, logistics, and application across health, beauty, and nutrition sectors. It serves as a one-stop resource for buyers, brand owners, and manufacturers seeking to understand the complete lifecycle and market integration of glutathione products.
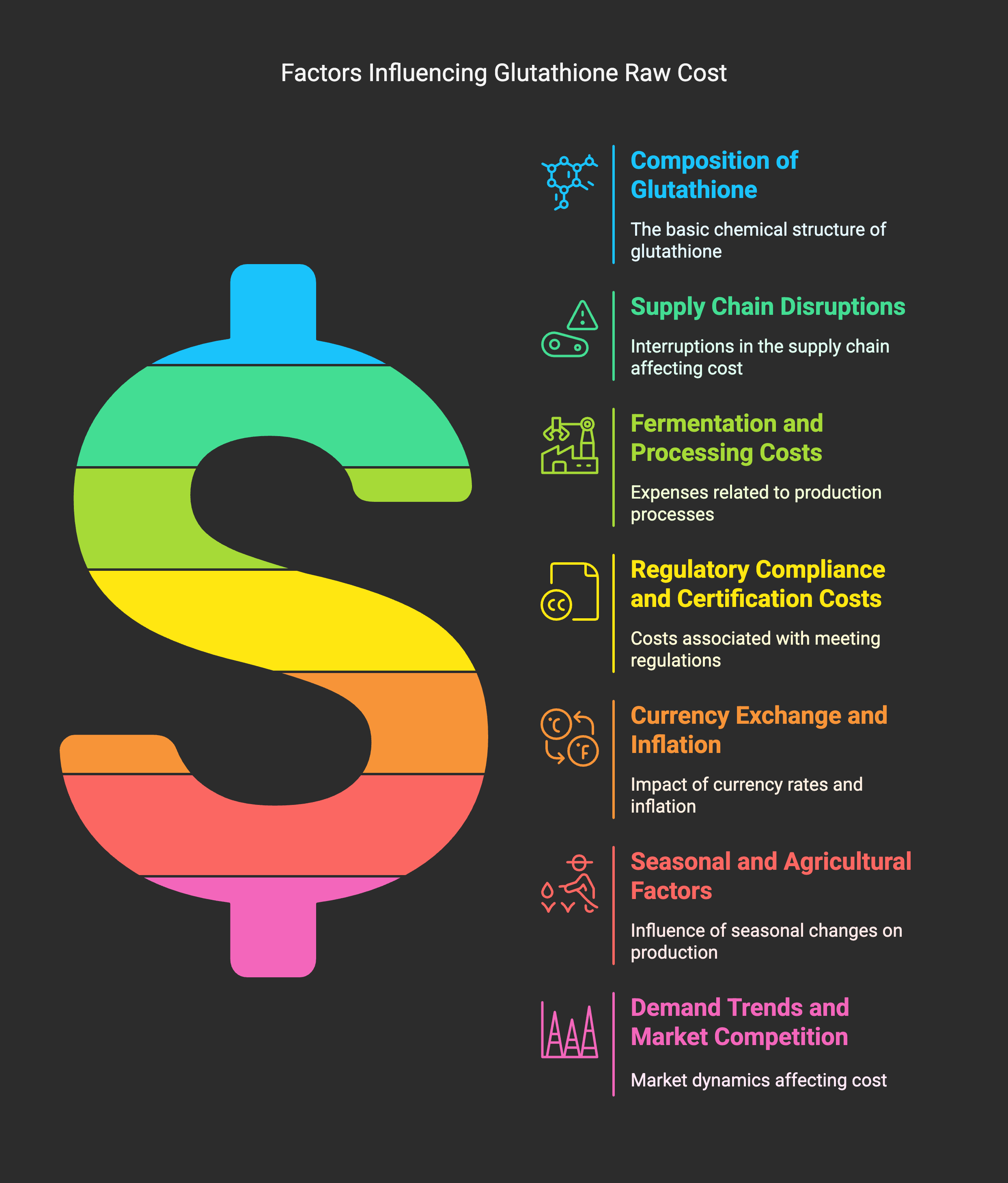
This article analyzes the key factors that influence fluctuations in glutathione raw material prices, including amino acid availability, fermentation input costs, energy prices, regulatory changes, and global supply chain disruptions. It also offers risk mitigation strategies such as supplier diversification and contract pricing models.

This article compares fermentation and chemical synthesis as the two main production methods for glutathione. It evaluates each based on purity, bioavailability, cost, sustainability, and regulatory fit. The guide helps formulators, manufacturers, and buyers choose the most appropriate method based on their product goals and market segment.
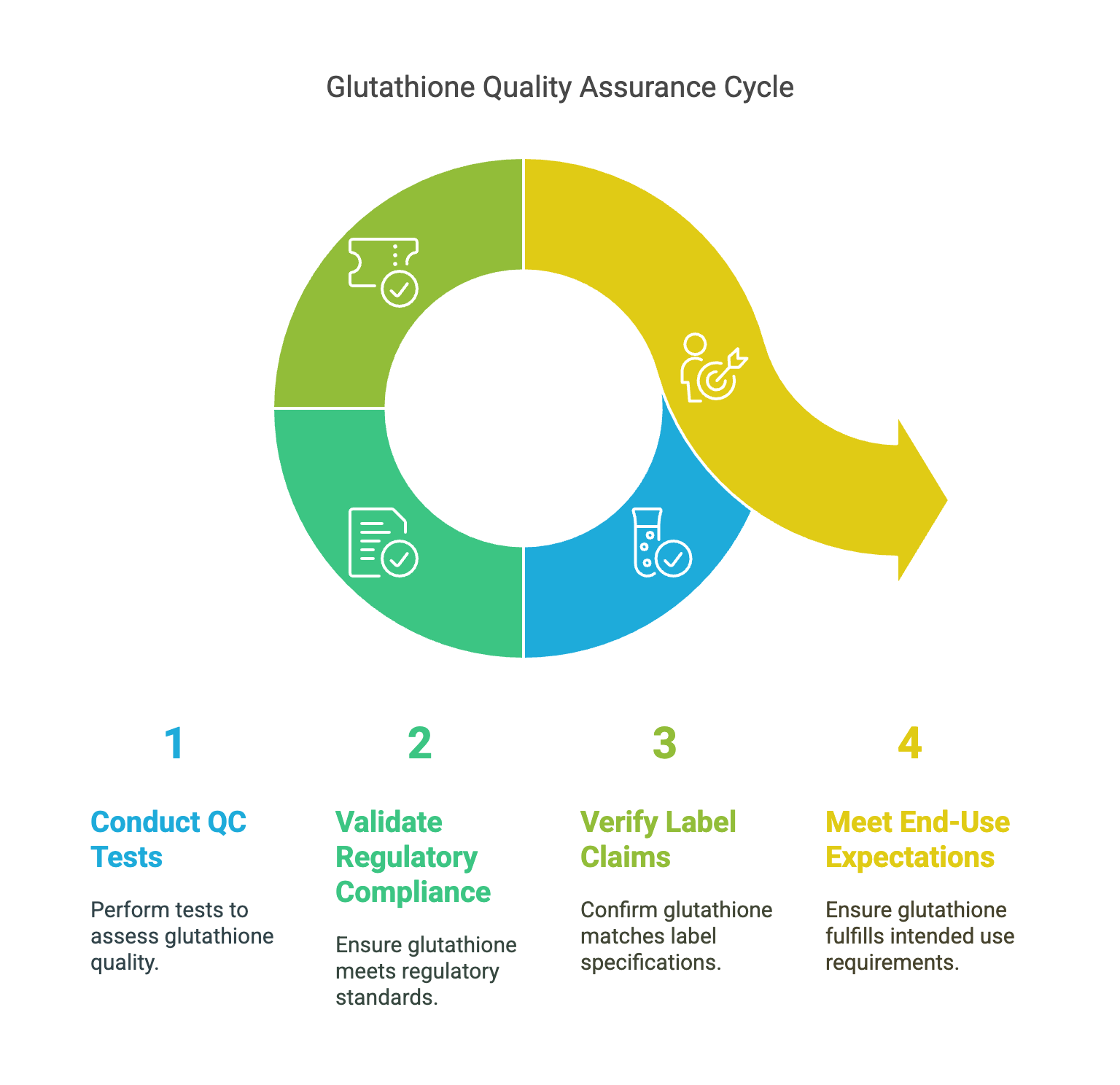
This article outlines the core quality control tests used to ensure the safety, purity, and consistency of glutathione powder. It explains key analytical techniques such as HPLC, ICP-MS, microbial limits, moisture analysis, and residual solvent testing. Aimed at manufacturers and bulk buyers, it highlights what to expect in a COA and how these tests protect product integrity.
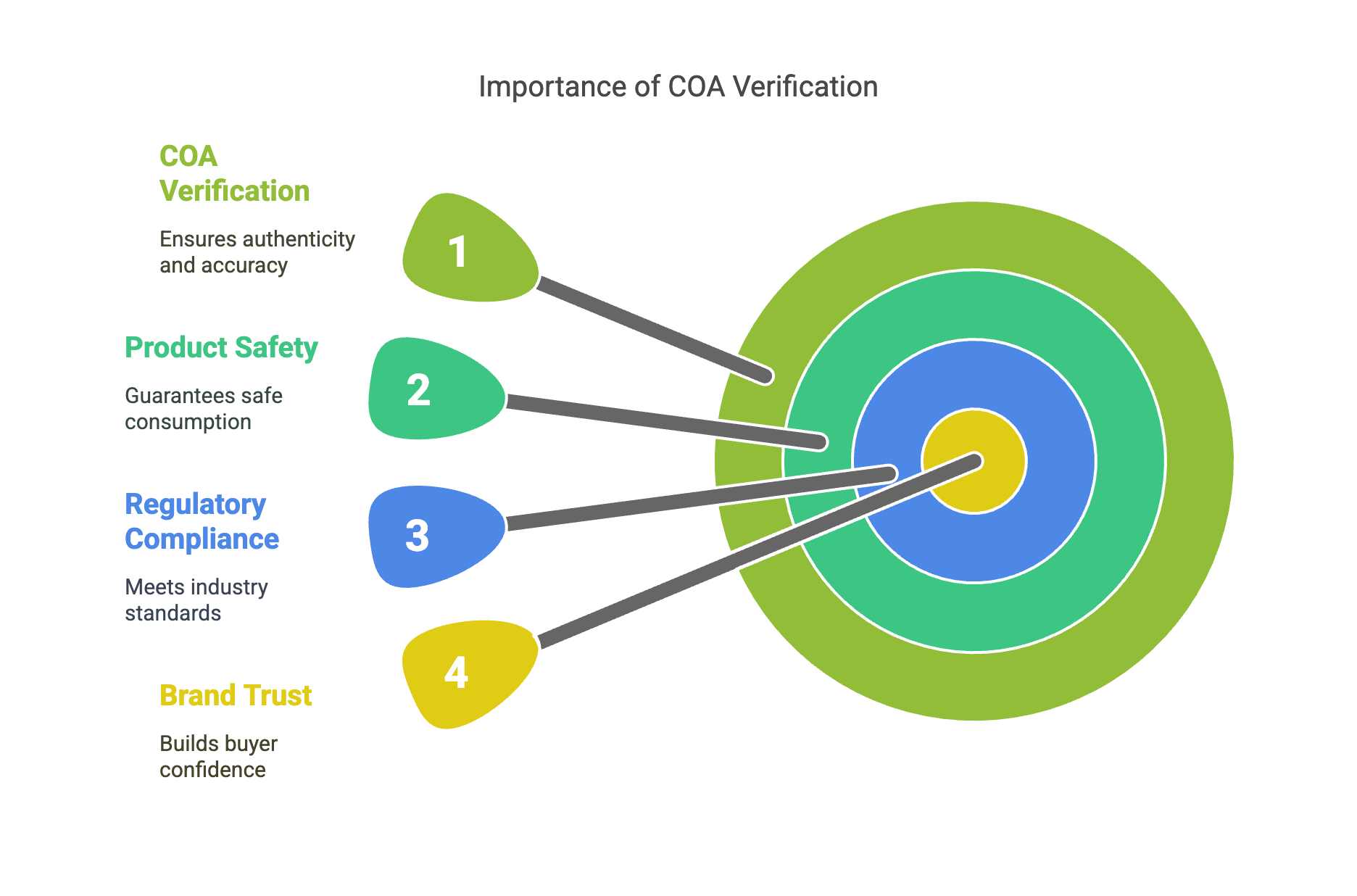
This article provides a step-by-step guide to verifying the authenticity of a Certificate of Analysis (COA) for glutathione powder. It covers key document elements, third-party lab credentials, specification matching, test methodology, and supplier communication. By following these practices, buyers can safeguard against counterfeits and ensure product quality and regulatory compliance.

We will contact you within 1 working day, please pay attention to the email with the suffix “forrest@oriherb.com”.