Introduction: Why Size Matters in Liposomal Delivery
Liposomal delivery systems are gaining momentum in the nutraceutical and pharmaceutical industries due to their ability to enhance the absorption of poorly bioavailable compounds. Among the key factors influencing the performance of liposomes, particle size plays one of the most critical roles. For B2B buyers, understanding how size impacts function can guide better formulation choices and help evaluate supplier claims.
This article explores the science of liposome particle size and its direct effect on absorption, tissue penetration, and product performance.
1. What Is Liposome Particle Size?
Liposomes are microscopic vesicles composed of phospholipid bilayers that surround an aqueous core. Their particle size, measured in nanometers (nm), can range from 50 nm to over 1000 nm depending on the production method.
Common classifications:
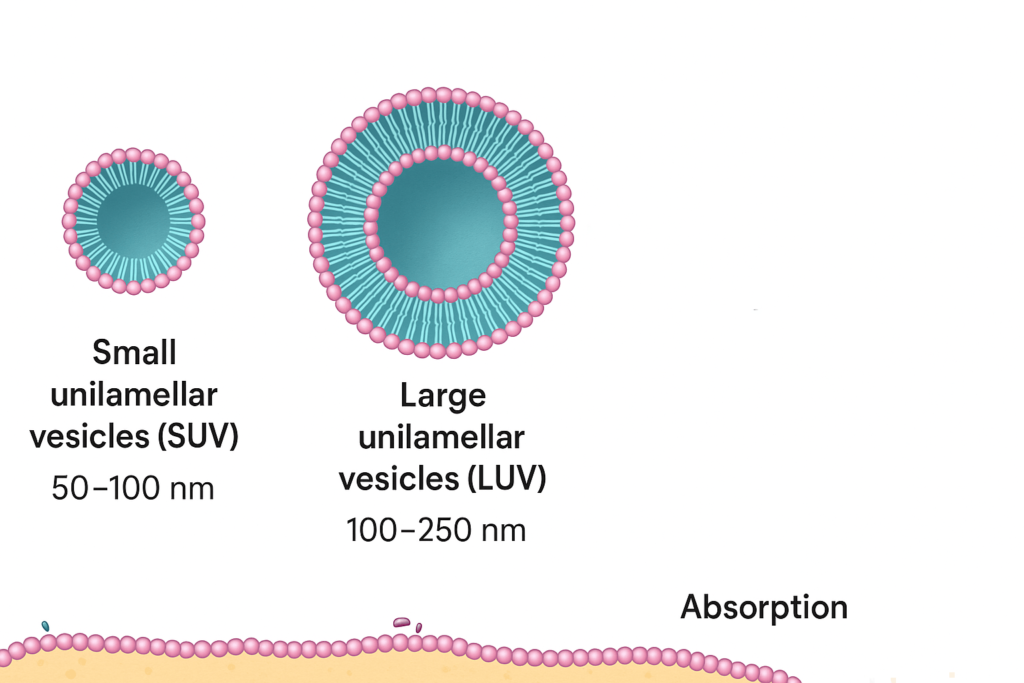
- Small unilamellar vesicles (SUV): 50–100 nm
- Large unilamellar vesicles (LUV): 100–250 nm
- Multilamellar vesicles (MLV): >250 nm
Smaller liposomes typically exhibit better absorption, longer circulation time, and improved cellular uptake due to their higher surface-to-volume ratio and ability to evade early degradation.
2. Why Smaller Liposomes Absorb Better
Liposomes smaller than 200 nm can more easily penetrate mucosal layers and intestinal epithelial cells, releasing their active compounds more efficiently at the target site.
Mechanisms of enhanced absorption include:
- Endocytosis by enterocytes
- Lymphatic transport bypassing first-pass metabolism
- Increased stability in gastric and intestinal fluids
Supporting Data: A 2022 study published in Pharmaceutics found that curcumin-loaded liposomes sized at 120 nm exhibited 2.4 times higher oral bioavailability than formulations sized at 400 nm.
3. The Dangers of Oversized or Unstable Liposomes
Liposomes larger than 400 nm are prone to aggregation and sedimentation, leading to poor absorption. These oversized vesicles are also more likely to be detected by the immune system and cleared from circulation quickly.
Risks of poor-quality liposomes:
- Low formulation transparency due to missing particle size data
- Clouding or sedimentation in liquid products, indicating poor stability
Expert Tip: Always request particle size data verified by dynamic light scattering (DLS) or transmission electron microscopy (TEM).
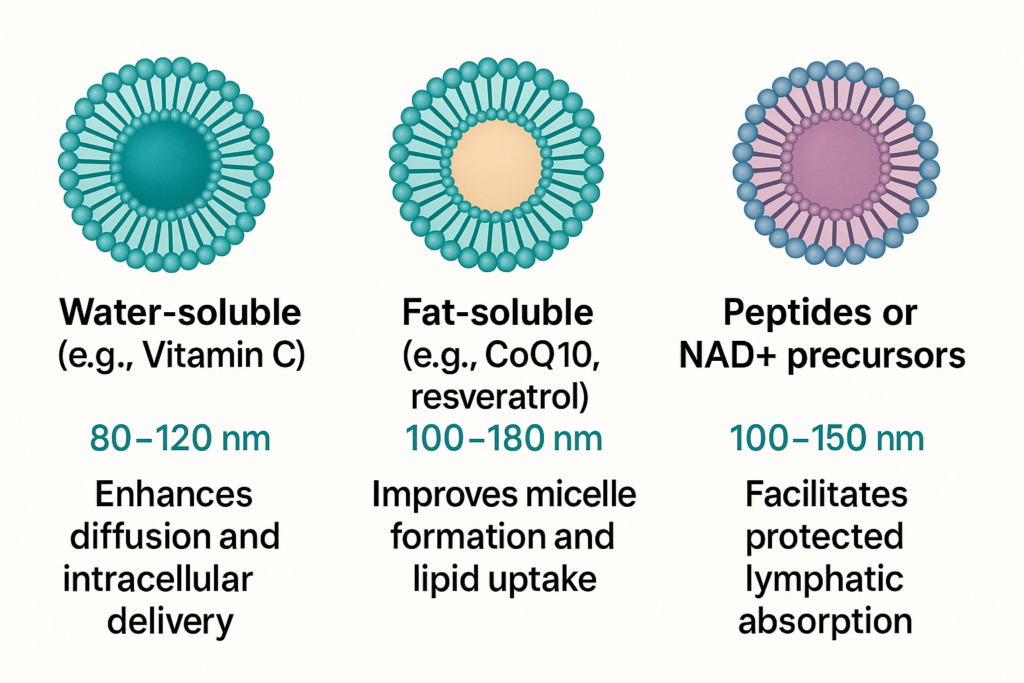
4. Ideal Particle Size by Ingredient Type
Different compounds benefit from different liposome sizes. Here’s a general guide to match ingredient types with optimal liposomal particle sizes:
| Ingredient Type | Ideal Liposome Size | Reason |
|---|---|---|
| Water-soluble (e.g., Vitamin C) | 80–120 nm | Enhances diffusion and intracellular delivery |
| Fat-soluble (e.g., CoQ10, resveratrol) | 100–180 nm | Improves micelle formation and lipid uptake |
| Peptides or NAD+ precursors | 100–150 nm | Facilitates protected lymphatic absorption |
5. How to Verify Liposome Size Claims
Reliable manufacturers are transparent about their testing practices and results.
What reputable suppliers provide:
- Batch-specific particle size distribution
- Polydispersity index (PDI), with values below 0.2 indicating high uniformity
- DLS or electron microscopy reports, often included in certificates of analysis (COAs)
Key questions to ask:
- What is your average particle size and PDI?
- How is consistency verified between batches?
- Are third-party test reports available?
Conclusion: Size Is a Strategic Decision, Not Just a Statistic
For B2B buyers and product developers, liposome size is not a trivial metric—it’s central to determining a product’s absorption potential and overall performance. By targeting smaller, consistent particle sizes and verifying claims with data, businesses can deliver superior liposomal formulations that meet consumer expectations and regulatory standards.
References
- Zhang, H. et al. (2022). Effect of particle size on the bioavailability of liposomal curcumin. Pharmaceutics, 14(4), 853.
- Mozafari, M. (2005). Liposome technology and applications. Cell Mol Biol Lett, 10(4), 711–719.
- Yadav, N. et al. (2021). Influence of liposomal size and composition on stability and performance. Int J Pharm, 597, 120306.