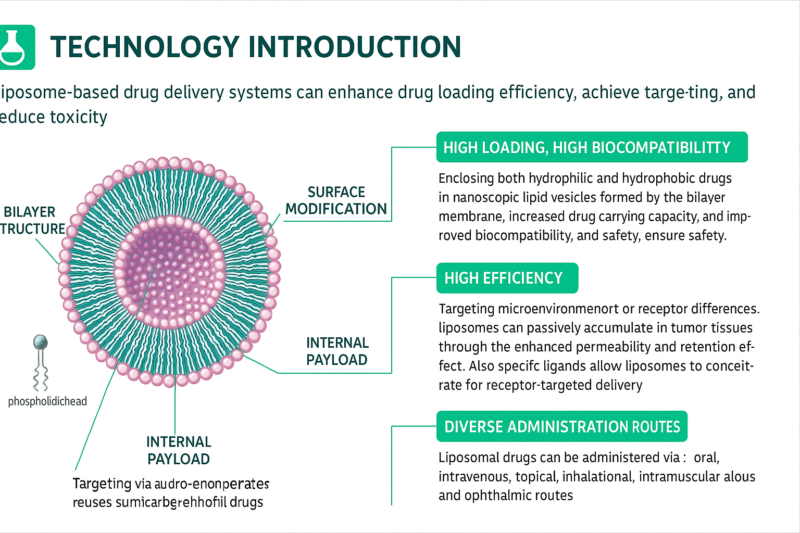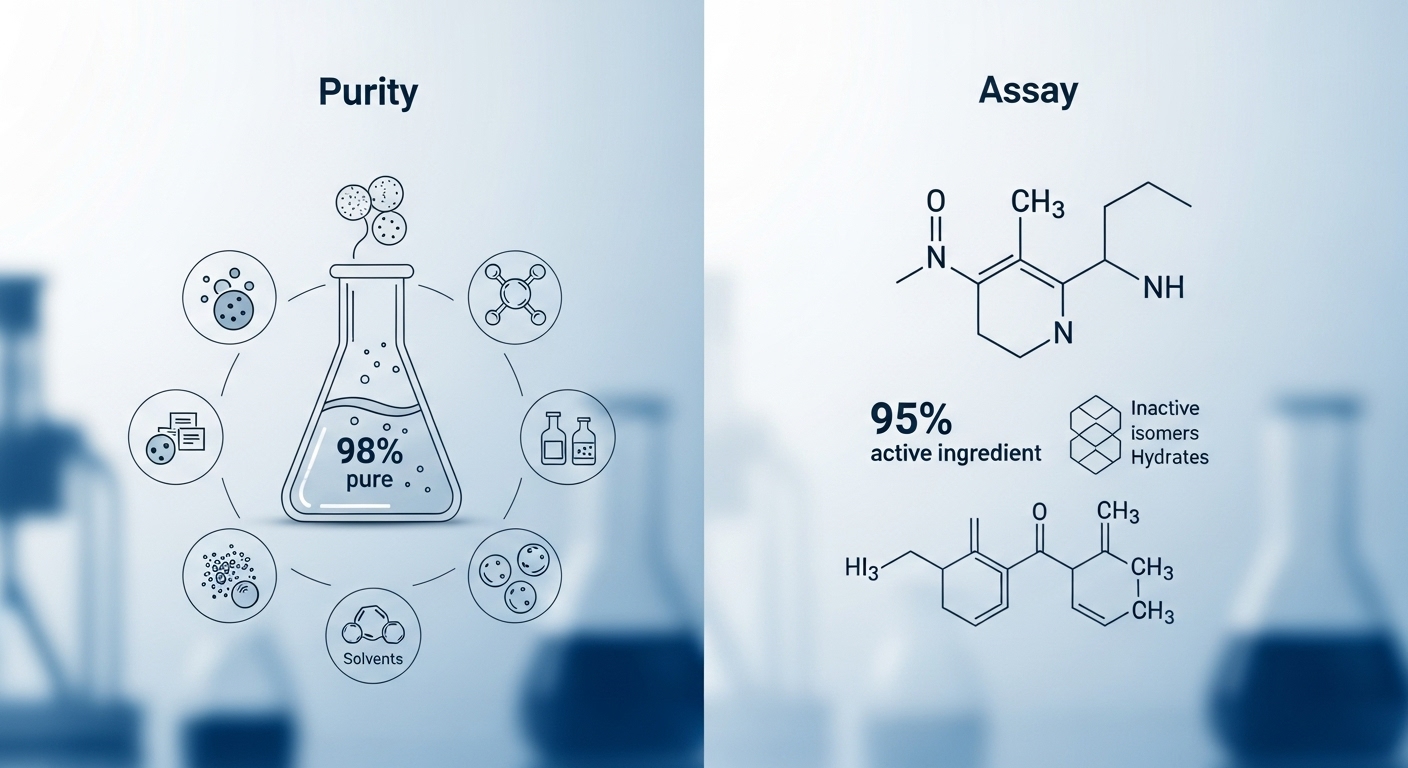
Purity vs. Assay: What’s the Real Measure of Quality in Chemicals and Pharmaceuticals?
In the pharmaceutical, chemical, and biotech industries, understanding the distinction between purity and assay is critical for quality control and regulatory compliance. Purity measures how free a substance is from impurities, while assay quantifies the actual amount of the target active compound. Although a product may be 99% pure, it could contain only 90% of the intended active ingredient due to degradation or the presence of inactive isomers. This article explains their definitions, calculation methods, analytical techniques, and real-world applications. It also explores how both metrics complement each other in formulation, manufacturing, and approval processes, helping industry professionals make informed decisions in material selection and product evaluation.