Introduction: The Rise of Liposomal Supplements
Liposomal technology has emerged as a cutting-edge delivery system in both nutraceutical and pharmaceutical industries, known for improving the bioavailability and effectiveness of active ingredients. As liposomal supplements continue to gain popularity, especially in B2B channels, one key question arises: Are all liposomes created equal? The short answer is no. Liposome quality varies significantly depending on raw material purity, manufacturing methods, particle characteristics, and testing standards.
This article offers a practical, data-driven guide for wholesalers, brand developers, and formulators to evaluate liposomal product quality and make better-informed sourcing decisions.
1. Liposome Structure and Composition: Foundation of Efficacy
High-quality liposomes mimic natural cell membranes using purified phospholipids arranged in a bilayer structure. However, formulations vary widely. Some use soy or sunflower lecithin with unclear phospholipid profiles, while others add synthetic emulsifiers or fillers that weaken vesicle integrity and reduce bioavailability.
Quality Indicators:
- Purified, non-GMO phospholipids
- Presence of cholesterol or plant sterols for bilayer stabilization
- Consistency in liposome structure (unilamellar vs multilamellar)
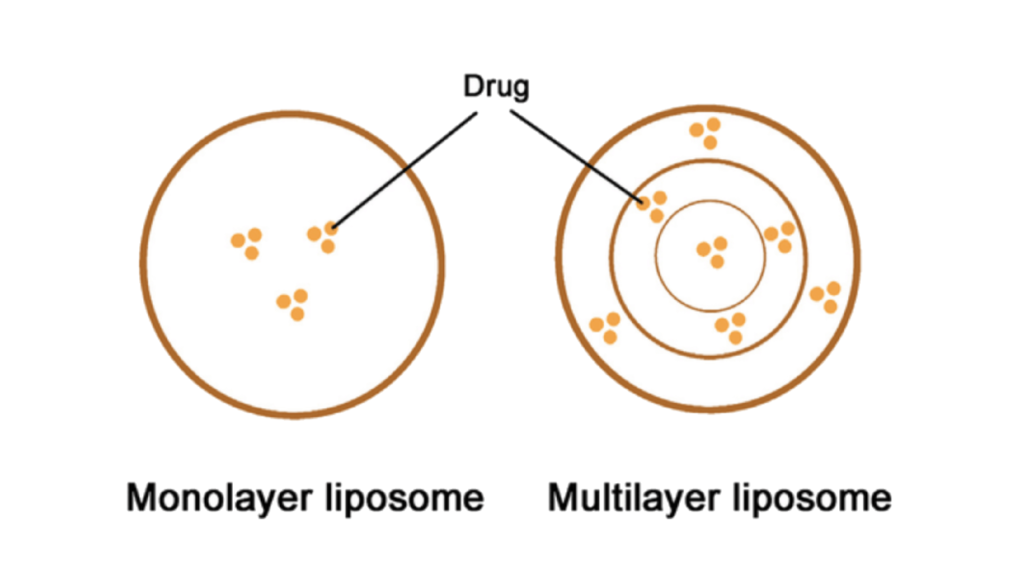
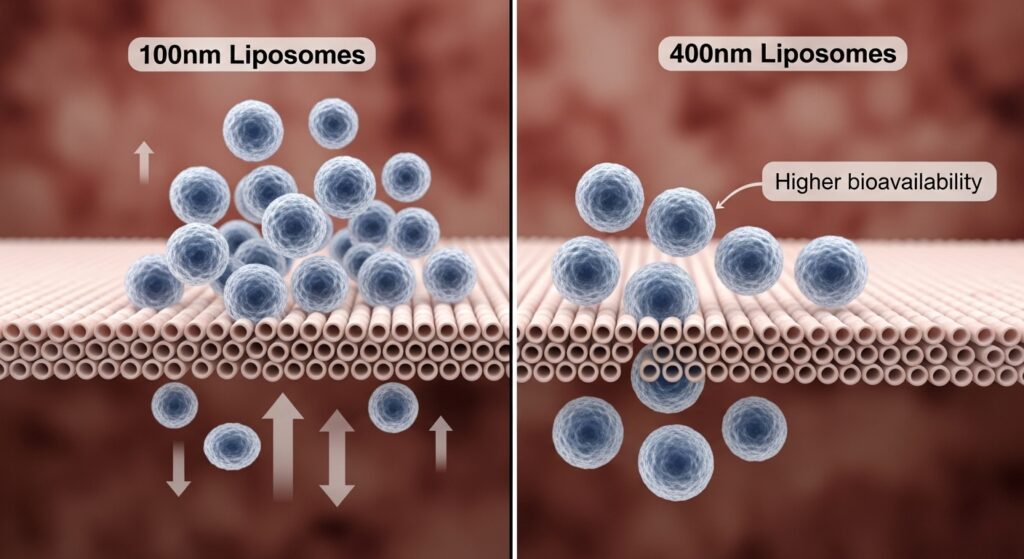
2. Particle Size and Distribution: A Gateway to Absorption
Particle size is directly linked to bioavailability. Research suggests that liposomes between 80–200 nm penetrate the intestinal lining more effectively and are more stable in biological environments.
Key Metrics:
- Target particle size: 80–200 nm
- Low polydispersity index (PDI < 0.2 preferred)
- Measurement via dynamic light scattering (DLS) or transmission electron microscopy (TEM)
Supporting Data:
A 2022 study in Pharmaceutical Nanotechnology found that liposomes under 200 nm showed 1.8× higher bioavailability for curcumin and CoQ10 than larger vesicles (>400 nm).
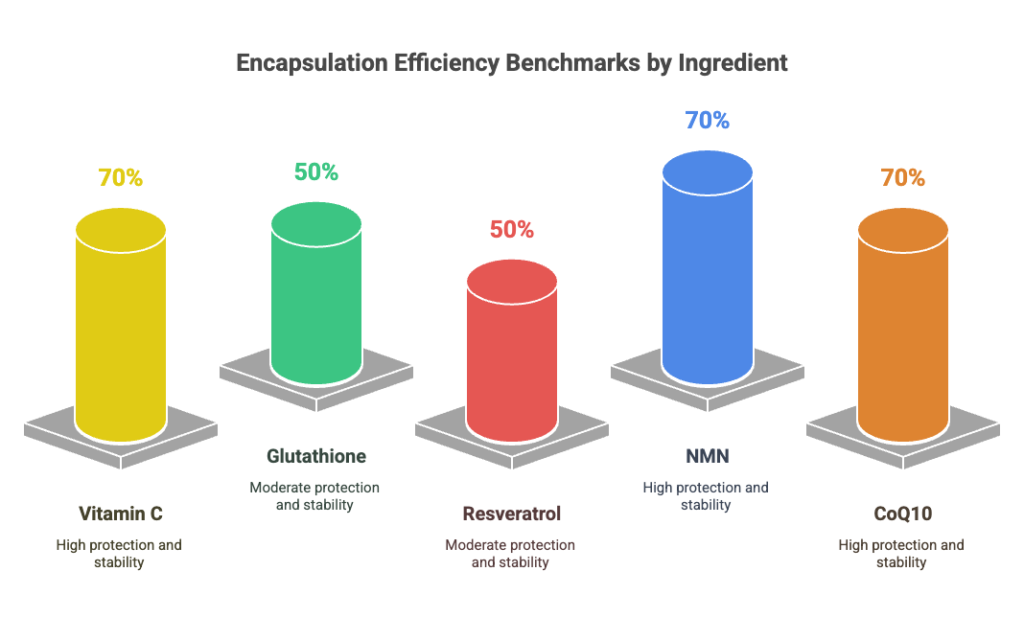
3. Encapsulation Efficiency: Measuring Real Payload Delivery
Encapsulation Efficiency (EE%) reveals how much active ingredient is actually enclosed within liposomes. Higher EE correlates with better protection, delayed degradation, and reduced ingredient waste.
Benchmarks by Ingredient Type:
- Water-soluble: >90% (e.g., glutathione, vitamin C)
- Fat-soluble: 70–85% (e.g., resveratrol, NMN, CoQ10)
Red Flag: EE < 50% may indicate poor formulation or vesicle breakdown during production.
Comparative Insight: Some liposomal NMN products on the market have EE as low as 45%, whereas optimized formulations reach up to 88%, significantly impacting absorption rates.
4. Manufacturing and Quality Control Standards
A major differentiator in liposomal quality is the manufacturer’s adherence to GMP standards and testing rigor. Production conditions—such as pH control, sterilization, and nitrogen flushing—greatly affect liposome stability.
Sourcing Questions to Ask:
- Is GMP or ISO 22000 compliance documented?
- Are third-party lab results available for EE%, particle size, and microbial testing?
- What type of equipment and batch traceability is in place?
5. Shelf Stability and Packaging: Preserving Potency
Liposomes are prone to oxidation, pH degradation, and clumping if not properly packaged. Premium products are packaged in UV-resistant containers with vacuum sealing or nitrogen flushing.
Stability Best Practices:
- Opaque, UV-resistant glass or multilayer pouches
- Temperature-stable shipping methods
- Addition of antioxidants like tocopherols (vitamin E)
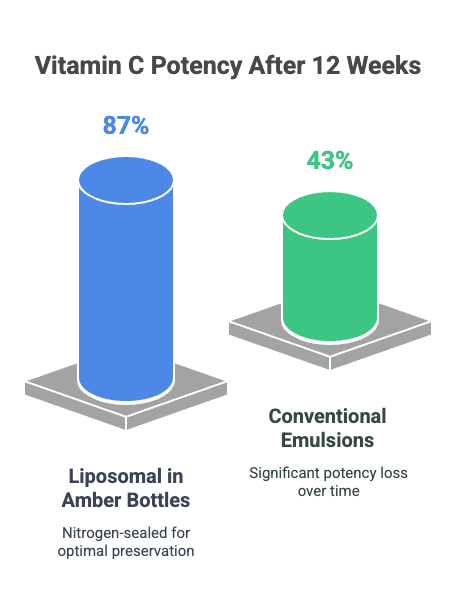
Case Study:
A 2021 Food Chemistry study found that liposomal vitamin C stored in nitrogen-sealed amber bottles retained 87% potency after 12 weeks at 25°C, while conventional emulsions dropped to 43%.
Conclusion: Invest in Liposome Quality That Delivers
For B2B supplement brands and functional ingredient buyers, selecting high-quality liposomal products is more than a trend—it’s a strategic necessity. Variables such as phospholipid source, particle size, EE%, and packaging can profoundly affect product performance and customer satisfaction. Scrutinizing your supplier’s practices and asking the right questions ensures that you’re delivering not just a product—but bioavailable, trustworthy innovation.
References
- Mozafari, M.R. (2005). Liposomes: An overview of manufacturing techniques. Cell Mol Biol Lett, 10(4), 711–719.
- Rakesh, R., & Sinha, V.R. (2018). Liposomal formulations: From concept to clinic. Int J Pharm, 547(1–2), 20–36.
- Li, Y. et al. (2022). Liposome size and oral bioavailability: A comparative analysis. Pharm Nanotechnol, 10(3), 178–185.
- Zhang, L. et al. (2021). Comparative stability of liposomal and emulsion-based vitamin C. Food Chem, 348, 129065.
- Zhao, F. et al. (2020). Influence of phospholipid composition on liposome stability. J Drug Deliv Sci Technol, 59, 101885.